Background:
Post-transplant lymphoproliferative disease (PTLD), a group of lymphoid disorders ranging from indolent polyclonal proliferation to aggressive lymphomas is a known complication following solid organ transplantation. The aim is to study the incidence, characteristics, predictive factors, management, and outcomes of PTLD after liver transplantation in particular.
Methods:
Following the PRISMA guideline, we performed a comprehensive literature search on PubMed, Cochrane Library, Embase, and clinicaltrials.gov from the past ten years on May 04, 2020. We used the MeSH terms of organ transplantation and lymphoproliferative disorders. The initial search revealed 1741 articles. We excluded all case reports, case series, pre-clinical trials, review articles, and meta-analysis. We found fifteen studies including retrospective observational and cohort studies. We extracted the data for baseline characteristics, the reason for transplantation, recipient & donor EBV status, immunosuppression used, type & stage of PTLD, organ system involved, duration between transplant and PTLD diagnosis, treatment, response to therapy, adverse effects of therapy and mortality.
Results:
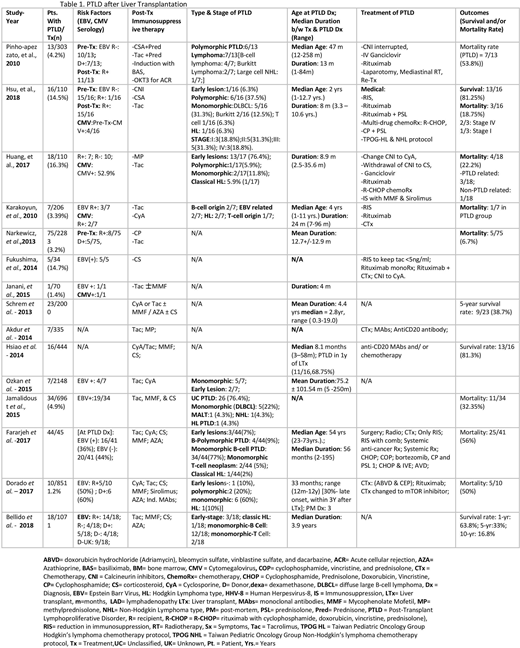
We included 15 studies with a total (n) of 10706 post-liver transplant patients. 294 (2.74%) patients developed PTLD as a complication, out of which 104 (35.3%) were males, 106 (36%) females, and 84 unknowns. Table 1. The incidence of PTLD in the pediatric group was 135/3116 (4.3%) whereas in the adult population it was 115/7545 (1.5%). Data from eleven studies show that out of 202 participants, 67 recipients were positive, 39 negative, and 18 donors positive for EBV infection at the time of transplant. Data on the EBV serostatus for the remaining 106 recipients was not known at the time of transplant. Data from two studies showed that 25/29 patients who later developed PTLD were seronegative for EBV prior to the transplant and 26/29 were reported to undergo seroconversion partly due to transplantation with EBV positive donors (7/13). Post-transplant immunosuppression was achieved with cyclosporine, tacrolimus, azathioprine, mycophenolate mofetil, sirolimus, prednisone, OKT3 for acute cellular rejection and induction with monoclonal antibodies. Data from four studies conclude the median age at the time of PTLD diagnosis is 47.6 months in the pediatric population while 54 years in adults, overall ranging from 1 year to 73 years. Ten studies show the overall median duration from organ transplant to the diagnosis of PTLD is 23.5 months. The median duration for the pediatric population was 11.6 months from the data collected from 55 patients whereas five studies with 166 adult liver transplant recipients showed a median duration of 35.5 months. Histopathological types were diagnosed via biopsy samples, with monomorphic in 76 (25.8%), early lesions in 22 (7.4%), polymorphic in 19 (6.4%), and classic Hodgkin lymphoma like PTLD in 8 (2.7%) of the samples. Among the monomorphic type, Diffuse Large B-Cell Lymphoma (DLBCL) was the most commonly reported i.e. 10/50 (20%) of patients with monomorphic type in two studies. Treatment of PTLD consisted of reduction or cessation of the post-transplant immunosuppressive drugs, anti-CD20 antibody (rituximab), antiviral treatment with ganciclovir, and lymphoma treated with chemotherapy, radiotherapy, and surgical resection. Data from eight studies show a mortality rate of 61/214 (28.5%) with Huang, et al. reporting ¾(75%) of total deaths due to PTLD progression. Two studies report an overall survival rate of 26/32 (81.3%) and five-year survival of 15/41 (36.6%).
Conclusions:
Our analysis shows the incidence of PTLD after liver transplant is low with no significant gender predominance but a difference in the incidence was observed with significantly higher rates in the pediatric group as compared to the adult population. The most common biopsy proved histopathological type was monomorphic, with the least common type being Hodgkin lymphoma like PTLD. Among the monomorphic, DLBCL was the most common subtype. After liver transplantation, the development of PTLD is observed earlier in pediatric patients in comparison to adult recipients. EBV naive patients prior to liver transplantation are at higher risk for seroconversion post-transplant if transplanted with EBV positive donors and hence at increased risk of PTLD development.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal